Discussion on transformation and application of purified water for solid preparation production
Part 1
Introduction
Process water is mainly divided into drinking water, purified water, water for injection and sterilization water for injection. The basic purpose of the preparation of process water is to control the particle load and microbial load in the production process. Common water quality indexes of process water include physical indexes and chemical indexes [1].
The pharmaceutical dosage forms produced by our company mainly include solid (tablets, capsules, granules) and oral syrups, and the dosage forms determine that our process water mainly involves drinking water and purified water. In the daily monitoring items of purified water, the physical indicators mainly include: properties (odor, taste, color), electrical conductivity, non-volatile matter, etc. The main chemical indicators are: pH, chlorine, nitrate, nitrite, total organic carbon, endotoxin, microbial limit, chemical oxygen demand and so on.
Purified water in the production can participate in the entire solid preparation and oral syrup production process, can be used as solvent, ingredients, experimental water, equipment cleaning water, etc., purified water is a very important public system. Our purified water preparation process is two-stage reverse osmosis, the specific process flow is: raw water → raw water pressure pump → multi-media filter → activated carbon filter → water softening device → security filter → primary reverse osmosis →pH regulation → intermediate water tank → secondary reverse osmosis → purified water tank → pure water pump → microporous filter → UV lamp sterilizer → each water point.
Part 2
Introduction to purified water preparation system
The purified water system is a very critical public system, which plays an important role in the whole management of GMP. There are two main ways to prepare purified water: two-stage reverse osmosis and first-stage reverse osmosis +EDI. Reverse osmosis is to add a certain pressure on the side of the high concentration, so that water flows from the side of the high concentration to the side of the low concentration. EDI, also known as continuous electron desalting, combines electrodialysis technology and ion exchange technology to make ion directional migration under the action of electric field to achieve the purpose of deep water purification and desalting.
Reverse osmosis +EDI combination purified water, better water quality, compact equipment, no acid and alkali pollution, but EDI equipment investment is large; The two-stage reverse osmosis system has the advantages of simple assembly, low operating cost and mature and stable technology. Combined with the actual situation, our company adopts a two-stage reverse osmosis preparation process, the arrangement type is "7+4" mode. That is, the primary reverse osmosis uses 7 Hydern RO membranes, the secondary reverse osmosis uses 4 Hydern RO membranes, and the pure water yield per hour is 1 t. Both the intermediate tank and the purified tank are SUS304 stainless steel 1 t storage tanks. Since the system was put into use in 2007, the daily maintenance is in place, the overall operation is good, the purified water meets the internal control standards, and the water production and storage can meet the daily drug production needs.
Part3
Reconstruction background
In recent years, our company's oral syrup sales orders have increased year by year. The volume of the original syrup preparation tank is 400 L. According to the demand of the sales order, the 400 L preparation tank is replaced with 1 000 L. During production scheduling, tanks and clean rooms are cleared immediately after preparation, followed by preparation of subsequent batches. Such continuous production can effectively increase production, but the demand for purified water increases a lot. The amount of purified water stored and produced can not guarantee the amount of pure water required for production after the transformation.
Considering the factors such as cost input, actual water demand and renovation period, it is decided to increase the water storage capacity of purified water, reserve enough buffer time, prepare water production without major changes, and meet the demand for large instantaneous water consumption by increasing the circulating water storage capacity.
Once the modification plan has been implemented, the parts of the previous preparation system that need to be improved are modified to ensure that the system continues to meet the requirements of the latest version of GMP and quality management concepts.
The problems that need to be improved are summarized as follows:
1) Insufficient capacity of water storage tank. During peak hours of purified water consumption, the water produced and stored in the system cannot be normally supplied to each use point.
2) Aging of RO membrane shell. RO membrane shell is made of stainless steel, one shell and one core installation form, both sides of the head has been obviously aging, the sealing performance is reduced.
3) Prepare the corrosion phenomenon of the system support. The support of reverse osmosis equipment is made of galvanized square pipe, which has been corroded and does not meet the hygienic and clean requirements.
4) Microporous filter. The 0.22μm microporous filter installed between the purified water storage tank and the UV lamp sterilizer was the conventional installation method many years ago, but in recent years, the quality management concept has been further improved to consider the problem more comprehensively. If the microporous filter is not cleaned, disinfected or replaced in time, it is easy to cause secondary pollution of purified water.
5) The pipe layout of the preparation system is messy. The flow of each medium cannot be clear.
6) There is no flow sensor in the return water main pipe. No flow sensor is installed, which can not reflect the flow and velocity parameters of purified water in the pipeline, and can not conduct real-time dynamic monitoring of the system.
7) Electrical control aging. The service life of the electronic components in the electric control box in the reverse osmosis device is too long, the aging is obvious and the configuration is slightly lower, the degree of automation is low, and the system can not be better served.
8) The size and structure of the cleaning tank are unreasonable. The volume of the chemical cleaning tank is 500 L, which is configured too large to occupy the indoor space of the purified water station, and it is not necessary to use such a large volume; And the water tank is flat bottom, no sewage valve, the use is not convenient.
9) The system has no online conductivity sensor. The on-line conductivity sensor is not installed in the system, and the conductivity of important points cannot be monitored in real time, and the water quality is not reflected in time.
10) Ordinary respirator. The main function of the respirator is to isolate the influence of external particles and microorganisms on the water quality of the tank, the general aperture is 0.22μm, and the material is tetrafluoroethylene PTFE. Our primary and secondary water storage tanks are installed with ordinary breathing apparatus, which has certain quality risks. The process flow of purified water before transformation is shown in Figure 1.
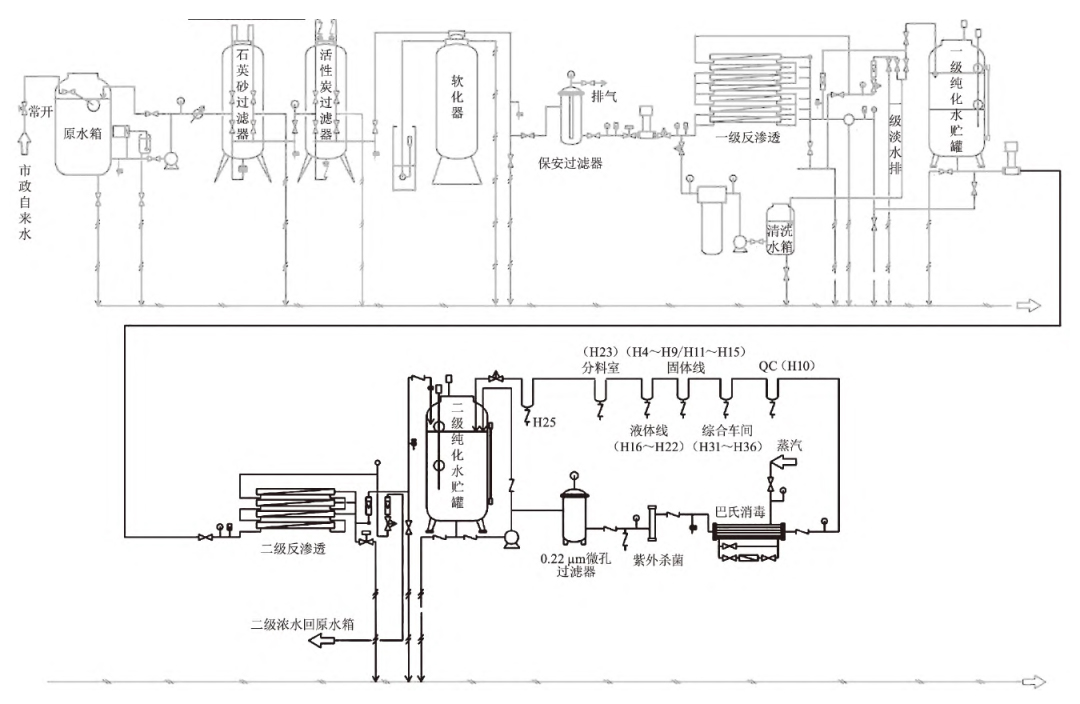
FIG. 1 Process flow of purified water before transformation
Part 4
Reconstruction scheme
According to the actual demand of purified water and the various factors of the preparation system to be further upgraded, the transformation scheme of purified water was formulated.
1) Increase the volume of water storage tank. In order to meet the maximum peak consumption requirements, it is calculated that the volume of the storage tank can be increased to 2 t according to the factors such as batch production water consumption and purified aquatic water consumption, which can be used as a buffer adjustment during the peak water consumption. The original 1 t pure water storage tank is still used, and 1 t pure water storage tank with the same specifications (tank height, tank bottom, diameter, volume and other parameters) is added side by side, and the total water storage volume can reach 2 t. The advantages of increasing the water storage volume in this way are: the transformation period can be shortened, the cost is lower than that of replacing the 2 t storage tank, the tank can effectively disperse the load on the floor after the water storage (the floor is the prefabricated board in the 1980s), and the tank height is suitable. In the later commissioning, it is necessary to adjust the inlet and outlet water flow of the two tanks to ensure that the dynamic volume of the two tanks is basically the same, which is convenient for daily use and quality management.
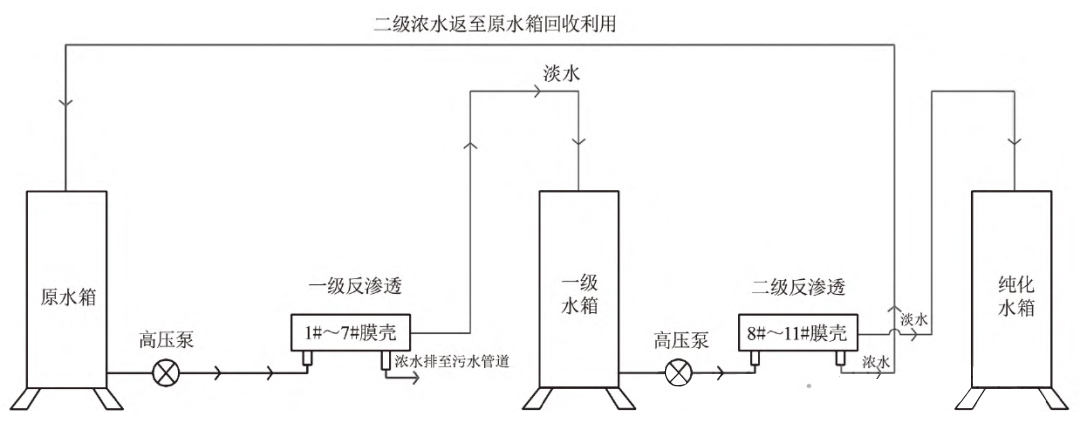
FIG. 2 Arrangement structure of RO film before modification
2) RO membrane shell replacement. The RO membrane shell is replaced with glass fiber reinforced plastic and installed with one shell and two cores. There are 11 original RO membranes, and the primary RO membrane and secondary RO membrane are arranged in 7:4 mode, as shown in Figure 2. This transformation uses 6 glass steel shells, that is, 12 RO films, of which 4 glass steel shells for primary reverse osmosis count 8 RO films, and 2 glass steel shells for secondary reverse osmosis count 4 RO films. The primary RO membrane adopts a 2:1:1 structure arrangement, and the secondary RO membrane adopts a 1:1 structure arrangement, as shown in Figure 3. That is, raw water is transported to the 1# and 2#RO shells through vertical high pressure pumps to produce fresh water into the primary water storage tank; 1# and 2# produce concentrated water into the 3# shell, and produce fresh water into the primary water storage tank; 3# concentrated water enters the 4# shell, producing fresh water into the primary water storage tank; The concentrated water produced by # 4 is discharged into the sewage pipe and no longer recycled; The water in the primary storage tank is transported to the 5#RO shell through the vertical high pressure pump to produce fresh water into the purified water storage tank; 5# concentrated water enters the 6# shell, producing fresh water into the purified water storage tank; 6# concentrated water is discharged into the original tank, and then filtered for recycling. By changing the arrangement of RO membranes, the water production rate of primary RO membranes can be increased from 50% to 75%, and the water production rate of secondary RO membranes can be increased from 75% to 85%.
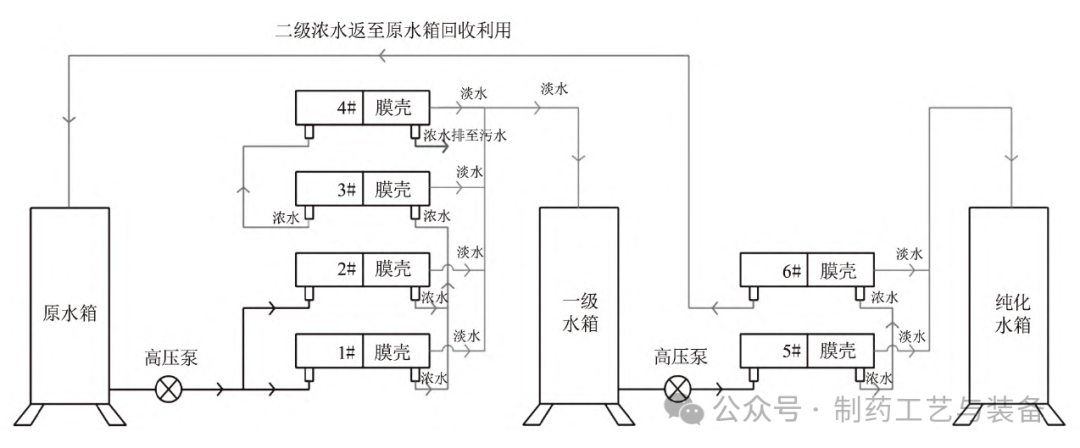 FIG. 3 Arrangement structure of RO film after modification
FIG. 3 Arrangement structure of RO film after modification
3) Prepare system support replacement. The bracket is made of SUS304 stainless steel material, and then the RO membrane shell, pipeline, high pressure pump, instrument, operation panel and other accessories are fixed and attached to it.
4) Cancel the microporous filter. With the further improvement of the quality management concept, consider the problem more comprehensive, the original installation of 0.22μm microporous filter between the purified water storage tank and the ultraviolet lamp sterilizer, such as daily operation and maintenance can not be cleaned, disinfected or replaced in time, it is easy to cause secondary pollution of purified water, increase the quality risk, so remove the microporous filter, no configuration.
5) Piping optimization of the preparation system. The indoor space of the purified water station is limited, and the placement of facilities and pipeline connections are optimized and adjusted to ensure the rational use of space and facilitate daily operation and production. According to Article 98 of Section 6 of Chapter 5 of China GMP (2010 edition), "Dead corners and blind pipes should be avoided in the design and installation of pipelines" [2], the welding conforms to clean pipeline welding standards and inspection standards. The media and flow direction in each pipeline are clearly marked.
6) Add a flow sensor to the return water main. It is installed at the end of the return water main pipe to monitor the flow rate of the return water pipe. Under normal circumstances, the flow rate of the end return water should be no less than 1 m/s to ensure that the water circulation of the purified water system is in a turbulent state, which can prevent the formation of biofilm. If the flow rate is too high, it will produce a flutter phenomenon. It is best to control the flow rate of backwater at 1 ~ 2 m/s. When the flow rate monitored by the flow sensor is less than 1 m/s, the alarm will be issued, and the operator will check the water use point in time and take corresponding measures.
7) Electrical control upgrade. The electronic components in the electric control box in the reverse osmosis device are updated, and multiple mode switching functions of "automatic" and "manual" are added. According to Article 100, Section 6, Chapter 5 of Chinese GMP (2010 edition), "The water quality of pharmaceutical water shall be regularly monitored" [2], online temperature measurement and online pH monitoring functions shall be added; Increase the monitoring function of purified water in tank.
8) Clean and replace the water tank. Taking into account the use requirements and space limitations of the cleaning water tank, the cleaning water tank is replaced with a PP water tank with a 200 L conical bottom and a drain valve is provided at the bottom.
9) Add an online conductivity sensor. In the pure water preparation system, 5 points of online conductivity sensors are added, which are raw water tank, primary RO film water production, secondary RO film water production and two purified water storage tanks. At the same time, a solenoid valve is installed on the conductivity sensor of the secondary reverse osmosis outlet point. When the conductivity value is higher than 2μS /cm, the bypass valve automatically opens, the water is automatically discharged, and the water is not entered into the purified water storage tank to ensure that the water quality entering the purified water storage tank meets the internal control quality standards.
10) Use electric heating respirator. During the daily operation of the purified water system, the purified water system will be pasteurized regularly, and the electric heating jacketed respirator is used, and it is equipped with a self-discharge port, which can keep the respirator dry, avoid condensation water accumulation on the filter membrane blocking the respirator, and avoid the risk of excessive microorganisms in the respirator.
The process flow of purified water after modification is shown in Figure 4.
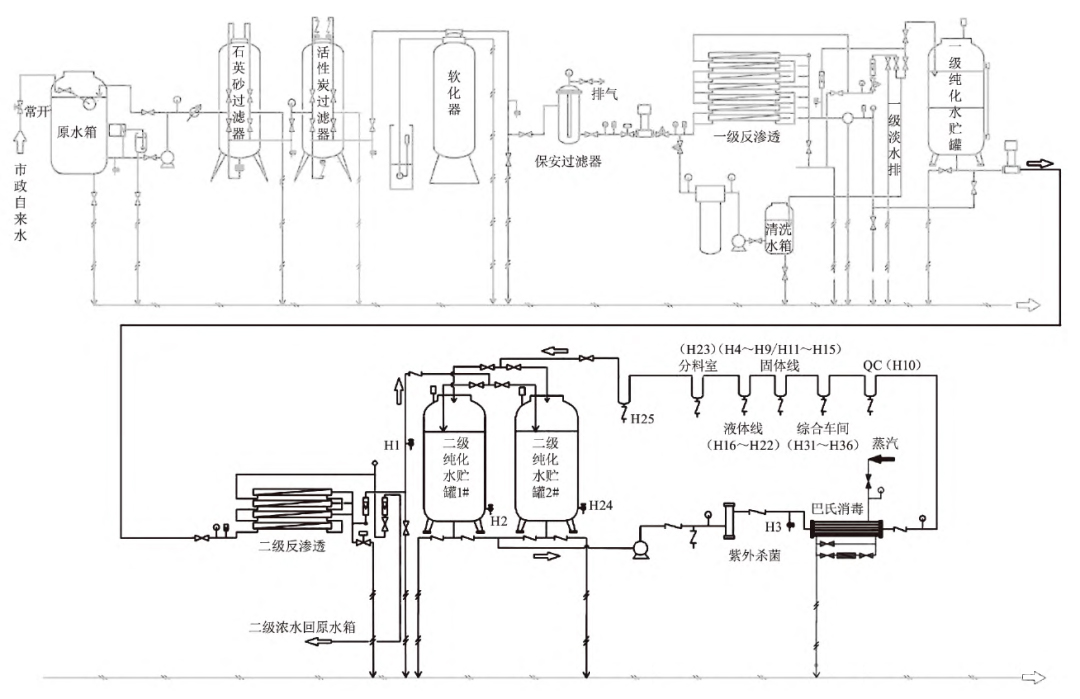
FIG. 4 Process flow of purified water after modification
Combined with the development trend of water system and the transformation time, cost and other costs, comprehensive consideration to determine the transformation plan, in line with the actual situation of our company, and can better meet the needs before the transformation.
Purified water preparation system is a more important part of the public system of the pharmaceutical factory, and the maintenance of the purified water system is also carried out in this transformation, such as replacing quartz sand, activated carbon, positive resin, and security filter elements. Before operation, the whole system is pickled, passivated and pasteurized to ensure that the water production and distribution system links meet the quality standards.
Modified or major maintenance equipment should be reconfirmed, therefore, the modified purified water system installation confirmation, operation confirmation and performance confirmation verification work, through verification to determine whether the equipment can continue to produce purified water that meets the quality standards, can supply the production water consumption, can ensure that the distribution system effectively transport and store purified water. The acceptance period of the purified water system is long. If the water quality meets the "Chinese Pharmacopoeia" and internal control standards, production activities can be carried out after the periodic verification report, and the later verification work will continue.
Part 5
Closing remarks
The preparation process of purified water system has a variety of ways and a variety of combinations, and the enterprise chooses the appropriate preparation process to ensure that the quality of the produced water meets the quality standards, and can also achieve the purpose of reducing cost and increasing efficiency and environmental protection.
Although the development of pharmaceutical production equipment and technology in China has been relatively mature, enterprises will still want to use the original equipment host as much as possible considering the cost of capital investment and transformation time. Under this premise, in order to ensure that the equipment continues to meet the requirements of laws and regulations and production processes, it is necessary for the technical personnel of the enterprise to customize a reasonable transformation plan, so that the equipment can better provide security for drug production, which also makes the enterprise and the entire industry put forward higher requirements and more expectations for the relevant practitioners.
Reference
[1] He Guoqiang. Pharmaceutical Fluid Process Implementation Manual [M]. Beijing: Chemical Industry Press, 2013.
[2] Regulation on Quality Control of Pharmaceutical Production (Revised in 2010) : Decree No. 79 of the Ministry of Health [A].


 FIG. 3 Arrangement structure of RO film after modification
FIG. 3 Arrangement structure of RO film after modification
