As a special commodity, drugs have the function of protecting and improving human health, which makes people pay more attention to the production environment of drugs and judge the health of drugs by the cleanliness requirements of production equipment. In the history of drug production, there are many drug quality accidents caused by incomplete cleaning [1]. The pharmaceutical packaging process mainly includes the following steps: product fluoroscopy, product printing or labeling, product packing in small boxes, product weighing, three-stage printing, three-stage testing, product wrapping or packing in boxes, traceability code coding, finished product packing, etc. Packaging as the last process of drug production, any link error will affect the quality of drugs, and even affect the life safety of patients, so the cleaning of production equipment is crucial. The discussion is of great practical significance to the cleaning of pharmaceutical packaging equipment and the safety of pharmaceutical production.

3.2 Selection of cleaning method
Paper, summaries, etc. Disassembly cleaning generally applies to the most difficult and time-consuming cleaning methods that cannot be used for manual cleaning or auxiliary tools. The parts that impede the most difficult parts need to be removed, and then cleaned by manual cleaning or auxiliary tools. The disassembly cleaning shall be cleaned by specialized maintenance personnel.
Because different packaging processes use different production equipment, the structure of each packaging equipment is also different, there are many difficult to clean parts, manual cleaning method has a certain difficulty, so for different cleaning environment, choose the right cleaning method is the basis of cleaning completion, in the cleaning process, it is also easier to find some equipment unpredictable damage, Timely detection and timely treatment, active maintenance, reduce cost losses; For the parts of the equipment that often produce debris and dust, the possibility of collecting dirt is reduced to a minimum by installing protective parts on the equipment, and the frequency of cleaning is reduced. For equipment parts of different materials, the cleaning method should be strictly cleaned in accordance with the material cleaning instructions of the equipment. On the one hand to ensure product quality without risk, does not affect the stability of equipment operation, on the other hand to extend the service life of equipment.
The cleaning method of the equipment should adopt the cleaning manual provided by the equipment supplier or professional suggestions on equipment cleaning, and optimize the selection based on the comparison of the cost and effect of consumables in actual applications, and then the production management personnel will organize the equipment cleaning SOP as a guiding document for the cleaning personnel.
3.3 Selection of cleaning agent
The cleaning difficulty mainly depends on the physical and chemical properties of the active ingredients in the dirt, such as adsorption, solubility, etc., and the target cleaning substances are determined based on the pharmacological effects of the active ingredients [4]. The cleaning agent should be able to effectively dissolve the residue, do not corrode the equipment, and itself can be easily removed, as harmless as possible to the environment, or can be treated harmlessly. When cleaning, we should try to choose a detergent with simple composition and exact composition [5]. According to the type of dirt selected cleaning agent, should also consider the concentration of cleaning agent, temperature, action time, physical properties, etc., these factors will affect the cleaning effect, can not be isolated to consider separately, for different parts of the equipment material, choose the appropriate cleaning agent. Cleaning agents or disinfectants should be managed by special personnel, special inventory, storage location should be selected away from the production area.
Commonly used cleaning agents are alcohol, gasoline, WD-40 rust remover, 530 cleaning agents, lubricating oil, etc., for different cleaning parts, choose to use non-corrosive cleaning agents for cleaning parts, neither affecting the normal use of equipment, nor affecting the quality of the product.
Equipment protective covers generally use plexiglass and acrylic materials, the cleaning method of plexiglass cleaning is: water with 30% medical alcohol (75%), can not use industrial alcohol, because alcohol is the killer of plexiglass, high concentration of alcohol will produce chemical reaction with the surface of plexiglass, resulting in corrosive damage to plexiglass. The shield of acrylic material can be scrubbed with a soft cloth using a small amount of soft detergent or water. Clean the PU conveyor belt with a rag dipped in a small amount of alcohol. The device display screen and camera use a 530 cleaner and dust-free cleaning cloth.
Equipment frame, guardrail, roller will generally use SUS304 stainless steel material, but due to the influence of unstable factors in the environment will also appear slight rust phenomenon, affecting the appearance of the equipment cleanliness, and SUS304 stainless steel material cleaning using a small amount of WD-40, and then use dry cloth wipe. The cleaning of precision equipment should be cleaned by professional cleaning personnel, and cleaned and corrected according to the cleaning cycle; For some parts directly in contact with the product, it is also necessary to carry out some disinfection and sterilization work to avoid secondary pollution during the use of the drug.
3.4 Formulation of cleaning cycle
Set up a special team to produce the maximum output per day, conduct risk assessment on the impact of pollution caused by various parts of the equipment during the production process, the frequency of equipment use, the amount of pollution accumulation, etc., on product quality, collect historical data and other cleaning knowledge, and formulate corresponding cleaning cycles for different equipment according to the assessment results, such as: 30 minutes, 1 day, 1 week, 1 month and other specified time cycle (specific cleaning cycle based on the risk assessment results), so as to ensure the stable operation of the equipment, reduce the impact of the equipment on product quality. As shown in Figure 2, the phase III offset (Angle) diagram, the phase III laser printer determines the cleaning cycle of the part by the impact of dust on the operation of the small box on the conveyor belt.
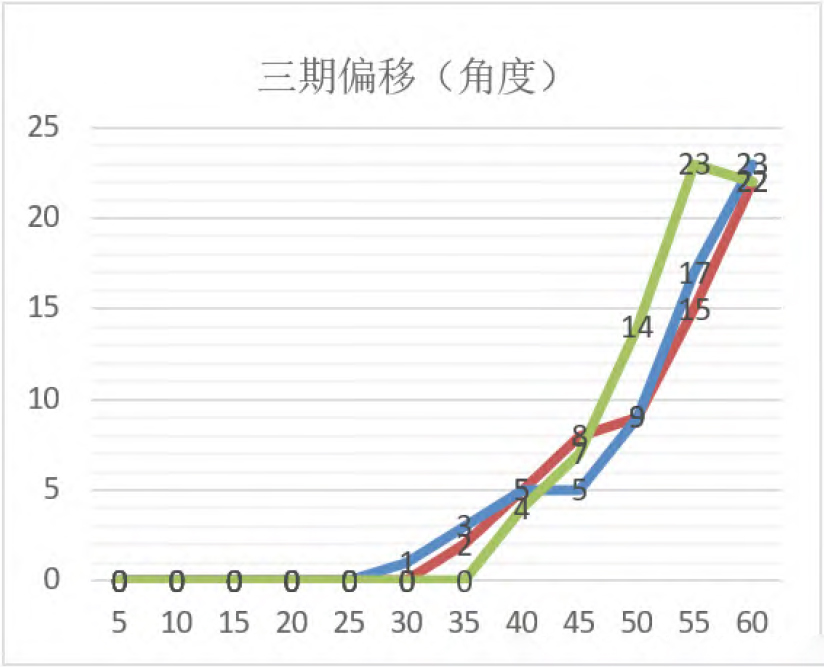
Figure 2
From the above three sets of data, it can be seen that after 30min, the content of the third phase of printed information is offset; After collecting 10 sets of data from the field, it was found that in the products with the third-stage offset, the printing box with the third-stage information offset of 2° was regarded as unqualified products, and should be immediately unpacked and repackaged. To sum up, the phase III printer should be cleaned immediately after 30min operation of the equipment, and the cleaning record should be filled in.
Check whether the equipment is within the cleaning validity period before use, and clean thoroughly when it is not within the validity period; Cleaning at the end of production, including: change shift, the end of the whole batch of production; Cleaning when changing the production variety; Clean the repair parts and contaminated parts of the equipment after maintenance.
3.5 Formulation of cleaning procedures
Article 72 of GMP regulations stipulates that operating procedures for cleaning, maintenance and repair of equipment shall be established, and corresponding operating records shall be kept. According to the results of cleaning verification, the corresponding equipment cleaning SOP and cleaning effect inspection mechanism shall be formulated. The documents require that the operation shall comply with the requirements of GMP regulations. The equipment cleaning SOP and cleaning effect inspection mechanism shall be revised, reviewed, approved, trained and implemented to make the cleaning process more streamlined. The corresponding contents of the cleaning procedure include:
(1) Safety preparation before cleaning
Before cleaning the equipment, power off the equipment to ensure that the components of the equipment are in a safe state and the safety of the cleaning personnel is ensured. The cleaning personnel of each equipment are fixed and professionally trained. For work involving mechanical parts disassembly and fastening, professional maintenance personnel to do.
(2) Cleaning procedures
The cleaning process shall be strictly carried out in accordance with the cleaning SOP and supervised by the inspectors. Special cleaning records shall be prepared for each equipment. The records shall be filled in according to the cleaning parts of the equipment and on time, and signed by the cleaning personnel, and confirmed by the inspectors after passing the inspection. There should be sufficient data and records in the cleaning confirmation to show that the equipment is clean and ready for use. The equipment cleaning SOP should include the following contents: (1) Safety preparation before cleaning; (2) Specify the cleaning methods for cleaning different parts of different equipment; (3) Specify which equipment specific parts to use the specified detergent; ④ Access and storage of cleaning tools and cleaners; ⑤ The internal inspection method of cleaning effect and the determination of cleaning standards.
3.6 Training of cleaning personnel
For pharmaceutical companies, the working attitude, operating skills and familiarity of cleaning personnel are very important to the final cleaning effect of equipment. Therefore, pharmaceutical companies must attach great importance to the training of cleaning personnel.
The cleaning effect is affected by various factors (such as the operator, the difference in operating conditions, etc.), and it cannot be guaranteed that all equipment surfaces can meet the required cleaning level, so the training of cleaning personnel greatly affects the cleaning effect. The department leaders and equipment engineers will formulate training documents after consultation, and the cleaning personnel will learn and practice. The cleaning personnel will be trained on a monthly basis. After the training is completed, the cleaning personnel will be assessed in the form of examination papers according to the training content.
3.7 Cleaning effect test method
Conduct risk assessment according to the impact of cleaning parts on equipment operation and product quality, determine inspection parts and cleaning degree, and formulate inspection mechanisms
(1) Cleaning of areas in direct contact with drugs
For the cleaning of some parts in direct contact with drugs, the cleanliness of the contact parts shall be inspected by the quality verification department, and the execution of the inspection shall be supervised by the quality assurance department. The test method is generally to use a cotton swab to dip the direct contact part, and QC through solution reaction detection and other methods to prove whether the part has an impact on the drug.
(2) Cleaning of the parts in indirect contact with the drug
For the cleaning of some parts in indirect contact with drugs, the test methods are generally: ① Visual inspection to check whether there is residue or color difference on the surface of the equipment after cleaning and drying. According to the understanding of GMP, after the production equipment is cleaned, there is no visible residue, which has become the minimum standard of equipment cleaning; The surface of the equipment is well polished, and there is a large contrast between the residue and the surface of the equipment. Generally, the visible residual amount is 4-20ug/cm2[7]. ② For those that cannot be determined by visual inspection, use the skin contact surface to perceive the cleaning effect or wipe sampling method.
3.8 Determination of cleaning standards
In the equipment cleaning, there are no harmful bacteria in the parts directly in contact with drugs, and no oil, dust, glass fiber, debris, and dirt in the parts of the equipment mechanical parts; There is no debris around the equipment, no gel and dirt on the conveyor belt and roller; Equipment protective cover and plexiglass surface without dust, stains, does not affect human vision. The surface of the device display is clear and does not affect the operator's operation identification. The camera is free of particles and dust, which does not affect visual verification. After the cleaning is completed, continuous testing is still needed to ensure the continuous effectiveness of the cleaning effect [6].