Clean workshop, also known as clean room, dust-free workshop, clean room, etc. Clean workshop is widely used in pharmaceutical and biological engineering, precision machinery, medical and health, food, electronic materials and other fields. In FED-STD-2, a clean room is defined as a room equipped with air filtration, distribution, optimization, construction materials and devices in which specific, regulated operating procedures are used to control the concentration of airborne particulates in order to achieve an appropriate level of particulate cleanliness.
The cleanliness degree of clean workshop and the continuous stability of pollution control are the core standards for testing the quality of clean rooms. Then the scope of detection in the clean workshop generally includes: clean room environment grade assessment, engineering acceptance testing, including food, health products, cosmetics, bottled water, milk production workshop, electronic products production workshop, GMP workshop, hospital operating room, biosafety laboratory, biosafety cabinet, ultra-clean workbench, dust-free workshop, aseptic workshop, etc.
Detection items: wind speed and volume, air exchange times, temperature and humidity, pressure difference, suspended particles, floating bacteria, settling bacteria, noise, illumination, etc. Specifically, you can refer to the relevant standards for clean room testing.
1, wind speed air volume air change number
The cleanliness of clean rooms and clean areas is mainly achieved by sending a sufficient amount of clean air to displace and dilute the particle pollutants generated in the room. For this reason, it is necessary to measure the air supply volume, average wind speed, air supply uniformity, air flow direction and flow pattern of clean rooms or clean facilities.
Unidirectional flow mainly relies on clean air to push and displace the polluted air in the room and the area to maintain the cleanliness of the room and the area. Therefore, the wind speed and uniformity of the air supply section are important parameters that affect the cleanliness. Higher, more uniform cross section wind speeds can more quickly and effectively eliminate pollutants from indoor processes, so they are the main detection items of concern.
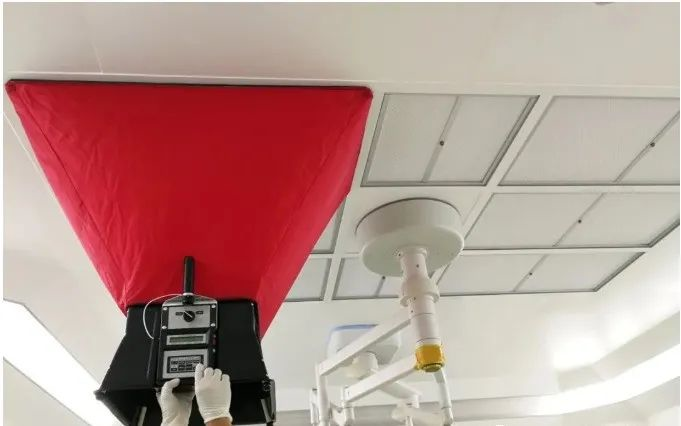
The non-unidirectional flow mainly relies on the clean air sent into the room to dilute and dilute the pollutants in the area to maintain its cleanliness. Therefore, the greater the number of air changes, the reasonable air flow pattern, the more significant the dilution effect, and the corresponding improvement in cleanliness. Therefore, the air supply volume and corresponding air exchange number of non-single-phase flow clean room and clean area are the main air test items of concern.
To obtain repeatable readings, the time average of wind speed at each measuring point is recorded.
The number of air changes: according to the total air volume of the clean room divided by the volume of the clean room
2. Temperature and Humidity
The measurement of temperature and humidity in clean rooms or clean facilities is usually divided into two grades: general test and comprehensive test. The first grade is suitable for completion acceptance testing in the empty state, and the second grade is suitable for static or dynamic comprehensive performance testing. This kind of test is suitable for the temperature, humidity performance requirements are more strict occasions. This test is performed after the air flow uniformity test and after the air conditioning system adjustment. At the time of this test, the air conditioning system was fully operational and all conditions were stable. At least one humidity sensor should be set up in each humidity control area, and the sensor should be given sufficient stability time. The measurement should be suitable for the purpose of actual use, and the measurement should be started after the sensor is stable, and the measurement time is not less than 5 minutes.
The purpose of this test is to verify the ability to maintain a specified pressure difference between the completed facility and its surroundings and between Spaces within the facility. This test works for all three occupancy states. This test needs to be performed regularly. The pressure difference test should be tested from high pressure to low pressure under the condition that all doors are closed, starting from the inner room furthest from the outside world on the plane layout, and then testing outward in turn; There are different grades of adjacent clean rooms (areas) with holes, and the hole should have a reasonable air flow direction and so on.
3, pressure difference detection requirements:
1) The measurement of static pressure difference is required to be carried out with all doors in the clean area closed.
2) On the clean plane, it should be carried out in the order of cleanliness from high to low, until it is detected to the room directly outside.
3) The measuring pipe mouth can be located in any place without the influence of air flow indoors, and the measuring pipe mouth surface is parallel to the air flow line.
4) The measured and recorded data should be accurate to 1.0Pa.
Pressure difference detection steps:
1) Close all the doors first.
2) Use the differential pressure gauge to measure the pressure difference between each clean room, between the clean room corridor, and between the corridor and the outside world.
3) Record all data.

Differential pressure standard requirements
Maintain the positive or negative pressure value of the clean room under test according to the clean room design or process requirements.
1) The static pressure difference between different levels of clean rooms or clean areas and non-clean rooms (areas) should not be less than 5Pa.
2) The static pressure difference between the clean room (area) and the outdoor should not be less than 10Pa.
3) For unidirectional flow clean rooms with air cleanliness grade more than 5 (100), when opening the door, the dust concentration of the indoor working surface at 0.6m inside the door should not be greater than the dust concentration limit of the corresponding level.
4) If the requirements of the above standards are not met, the fresh air volume and exhaust air volume should be re-adjusted until qualified.
4. Suspended particles
A. Indoor test personnel must wear clean clothes, no more than 2 people, should be located downwind of the test point and away from the test point, and should remain stationary. When performing the change point operation, the action should be light, and the interference of personnel on the indoor cleanliness should be reduced.
B, the equipment should be used during the calibration period.
C. "Zero clearance" of equipment before and after testing
D, in the unidirectional flow area, the selected sampling probe should be close to the isodynamic sampling, the wind speed into the sampling probe and the sampling air speed deviation should not exceed 20%. If this is not possible, the sampling port is positioned in the main direction of the air flow. For the sampling point of a non-unidirectional flow, the sampling port should be straight up.
E, the sampling port to the particle counter sensor connection tube should be as short as possible.

The sampling point is generally about 0.8-1.2m from the ground, and the point should be evenly and scientifically distributed, and the return air outlet should be avoided. For any small clean room or local air purification area, the number of sampling points shall not be less than 2, and the total sampling number can be obtained by opening two roots according to the area. The minimum number of sampling points corresponds to the number of suspended particle sampling points. The measuring point position in the working area is about 0.8-1.2m above the ground, and the measuring point position in the air supply outlet is about 30cm away from the air supply surface. The measuring point can be added to the key equipment or the key work activity range, and each sampling point is generally sampled once.
After the completion of all sampling, the petri dishes should be cultured in a constant temperature incubator for no less than 48 hours. Each batch of media should have a controlled experiment to check whether the media is contaminated.
5. Settling bacteria
The measuring point in the working area is about 0.8-1.2m above the ground. Place the prepared petri dish at the sampling point, open the lid of the petri dish, expose it to the specified time, then cover the petri dish, and put the petri dish in the constant temperature incubator for culture for not less than 48 hours. Each batch of media should have a controlled experiment to check whether the medium is polluted.
6. Noise
If the measuring height is about 1.2 meters from the ground, and the clean room area is less than 15 square meters, only 1 point in the center of the measuring room can be used; In the area of more than 15 square meters, it should also measure 4 diagonal points, 1 meter away from the side wall, and the measurement point is toward each corner.
7. Illumination
The measuring point plane is about 0.8 meters from the ground, and the measuring point is distributed according to the spacing of 2 meters. The measuring point of the room within 30 square meters is 0.5 meters from the side wall, and the measuring point of the room over 30 square meters is 1 meter from the wall.

Test standard
1) Clean Workshop Design Code GB50073-2001
2) Technical Code for Construction of Clean Surgery Department in Hospital GB 50333-2002
3) Technical Code for Biosafety Laboratory Building GB 50346-2004
4) Code for Construction and Acceptance of Clean Room GB 50591-2010
5) Test method for Suspended particles in clean room (area) of Pharmaceutical industry GB/T 16292-2010
6) Test method for planktonic bacteria in clean room (area) of Pharmaceutical industry GB/T 16293-2010
7) Test Method for Settling bacteria in clean room (area) of Pharmaceutical Industry